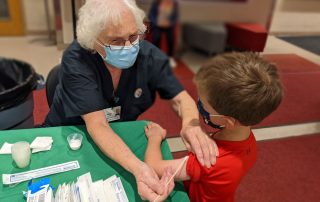
Westmont Hilltop School District hosts vaccine clinic for children
Last week, the Pfizer-BioNTech COVID-19 Vaccine was approved for children ages 5 through 11. Shortly after that, the Westmont Hilltop School District announced its plans to host a vaccine clinic with Highlands Health, in collaboration with the Cambria-Somerset Covid Task Force, on Monday, November 8. The clinic was open to Westmont and Ferndale school district [...]